There is a growing awareness in society, that safe-drinking water can not be taken for granted every time we open the tap. The public water services in today mega-cities are very sensitive to failures due to events such as power grid blackouts, social conflicts, unpredictable climatic events and others caused either by nature or man. This is why, knowing how to properly treat unsafe waters to make them suitable for drinking, may become a fundamental skill in the coming decades.
Introduction
It is paradoxical that while man insists on setting aside the pure and crystalline water of the word of our Lord Jesus Christ, to nourish himself of the contaminated waters of the modernist, atheistic and malicious currents that exclude religion; It is increasingly common to see in the news how suddenly, a big city, or sometimes entire countries, resort to water rationing, either because of good water scarcity or the impossibility of maintaining some flow rate in the public water services.
In the cities affected by scarcity, it is a common picture to see how citizens must strive and walk sometimes large distances carrying heavy containers, to get good quality water for the health of their families, who otherwise would lack of this resource, or they would have to settle for drinking contaminated water, harmful to the body’s health, and with the risk of contracting serious diseases related to the consumption of unsafe water.
These events that we always look from afar in our home, where perhaps we can open a tap “and that’s it”, should make us aware, both of the importance of this resource for the normal development of a society (or in other words, the potentiality for social chaos when absent), as of how unreliable the public water service can become when some types of events happen, either caused by nature or by man’s action, which can severely affect this service in just a matter of hours.
We believe, therefore, that having knowledge on how to properly treat unsafe waters to make them suitable for drinking will become a fundamental skill in the coming decades. Those who have the possibility should keep in their homes a printed or handwritten copy with information of this kind, so that it may be available offline. Besides, it is good to follow the advice of some environmental agencies from several nations, who recommend to have at home a minimum reserve of bottled water and dry foods, to better protect a family group during the first days of a natural disaster.
In Household Water Treatment Systems, as a general rule it is recommended to follow a multi-barrier approach:
Water source protection -> Sedimentation -> Filtration -> Disinfection -> Safe storage
This allows to make the most of the benefits of each technique to make the final result the best possible one.
Aeration
 Aeration brings water and air in close contact by exposing drops or thin sheets of water to the air or by introducing small bubbles of air, increasing the air content of water; this reduces the concentration of dissolved gases (such as carbon dioxide), which affect the odour or taste of water.
Aeration brings water and air in close contact by exposing drops or thin sheets of water to the air or by introducing small bubbles of air, increasing the air content of water; this reduces the concentration of dissolved gases (such as carbon dioxide), which affect the odour or taste of water.
Aeration also oxidizes dissolved metals such as iron (Fe), manganese (Mn), hydrogen sulphide and volatile organic chemicals (VOCs). Once oxidized, these chemicals fall out of solution and become particles that precipitate in the water that can then be removed by settlement or filtration.
Aeration is often the first major process at the treatment plant. During aeration, constituents are removed or modified before they can interfere with the treatment processes. Good aeration of the water is also important for slow sand filtration to be effective, especially if there is not enough oxygen in the surface water.
Aeration can increase the palpability of water by removing the flat taste. The amount of oxygen the water can hold depends primarily on the temperature of the water. (The colder the water, the more oxygen the water can hold).
Water that contains excessive amounts of oxygen can become corrosive.
How to aerate water
You can achieve aeration on a small scale by rapidly shaking a vessel 3/4 full of water. Aerate larger volumes of water by allowing them to trickle through one or more well-ventilated, perforated trays containing small stones. The scrubbing process caused by the turbulence of aeration physically removes dissolved gases from solution and allows them to escape into the surrounding air.
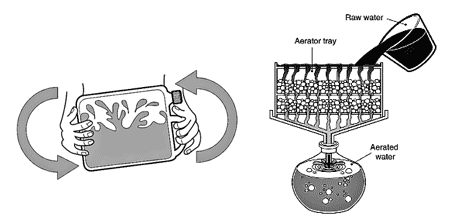
Chemicals removed or oxidized by aeration:
- Volatile organic chemicals, such as benzene (found in gasoline), or trichloroethylene, dichloroethylene, and perchloroethylene (used in dry-cleaning or industrial processes)
- Ammonia
- Chlorine
- Carbon dioxide
- Hydrogen sulfide
- Methane
- Iron and Manganese
Storage / Settlement
 Sedimentation is a physical treatment process used to remove small particles, such as sand, silt and clay, which make water cloudy.
Sedimentation is a physical treatment process used to remove small particles, such as sand, silt and clay, which make water cloudy.
Much of the suspended material can be removed by simply allowing the water to stand and settle for a period of time (e.g. 24 hs.). This can be done effectively in a small container such as a bucket or pail.
Microorganisms are often attached to the surface of particles in water, so removing these particles can also help to reduce the biological contamination. After several hours water collected from the top of the container will be relatively clear, unless the solids are very small (e.g. clay particles).
Storing water for just one day can result in the die-off of more than 50 per cent of most bacteria, but it should be stored for 48 hours to eliminate cercaria (snail larvae); conditions in storage vessels are usually not conducive to their survival! Longer periods of storage will lead to further reduction.
The three-pot treatment system exploits settlement and the death of pathogens during storage to improve the quality of raw water.
Settling, however, can only partially remove turbidity – which is a measure of the suspended solids. The time range may vary from one hour up to two days (the longer the better).
The sedimentation process can be accelerated by adding special chemicals (or natural products), also known as coagulants, to the water. These chemicals help the small particles in water join together forming larger clumps, making it easier for them to settle to the bottom of the container (See Coagulation – Flocculation for more details).
Coagulation – Flocculation
 If raw water contains a large amount of very fine suspended solids, coagulation and flocculation can be used to remove much of this material.
If raw water contains a large amount of very fine suspended solids, coagulation and flocculation can be used to remove much of this material.
In coagulation, a substance (usually in a liquid form), is added to the water to change the behaviour of the suspended particles. It causes the micro-particles, which previously tended to repel each other, to be attracted towards each other, or towards the added material. The water is then gently stirred to allow the micro-particles to come together and form larger clumps (flocculation) with specific weight greater than that of water, which can then be removed by sedimentation, settlement or filtration.
Three common chemicals used are aluminium sulphate, polyaluminium chloride (also known as PAC or liquid alum) and ferric sulphate.
Natural coagulants
Natural coagulants include some types of clay (e.g. bentonite) and powdered seeds of some trees. Native plants have been traditionally used in a number of countries in Africa and Latin America to help with sedimentation. For example, prickly pear cactus, dried and ground moringa seeds, broad beans and fava beans have all been used to help sediment water.
Fine Cloth Filtration
 This filtering method consists in pouring raw water through a piece of fine, clean, cloth to remove some of the suspended solids contained in the water. Cloth filtration is a long-practiced water treatment technique.
This filtering method consists in pouring raw water through a piece of fine, clean, cloth to remove some of the suspended solids contained in the water. Cloth filtration is a long-practiced water treatment technique.
A clean, cloth fabric can be used to strain particles out of water. Typically in south Asia, a ‘sari’ cloth is folded 7 to 8 times and used as a filter. Water is poured through the folded sari cloth and collected in a bucket underneath. Sari cloth filters are known to reduce the risk of cholera by filtering out particles and plankton which harbor the cholera bacteria.
 Huq showed that 99% of cholera parasites bound to planktonic copepods were removed by quadruple-folded sari cloth in Bangladesh (Huq, 1996). Similarly, the reduction in guinea worm incidence in Ghana and Sudan through use of the cloth filter is so pronounced that the GWEP expects world-wide eradication within the next few years (GW Wrap-up, 2008).
Huq showed that 99% of cholera parasites bound to planktonic copepods were removed by quadruple-folded sari cloth in Bangladesh (Huq, 1996). Similarly, the reduction in guinea worm incidence in Ghana and Sudan through use of the cloth filter is so pronounced that the GWEP expects world-wide eradication within the next few years (GW Wrap-up, 2008).
While this simple intervention has the possibility to target devastating diseases with large carrier hosts, diarrhea incidence is not likely to decrease through its use and concurrent treatment techniques are required.
To filter water through cloth:
-Fasten cloth to water storage vessel and tighten string, using cloth folded same side up each time.
-Rinse off filter after each use, with a final rinse of cloth filtered water and then leave cloth in the sun for decontamination.
-Clean cloth filter with soap regularly.
-If the cloth is dirty, additional pollutants may be introduced!
Charcoal Filtration
 Granular charcoal (or granulated activated carbon) can be used in filtration and is effective in improving the taste, odour and colour of the water. This system uses a bed of activated carbon to remove contaminants and impurities.
Granular charcoal (or granulated activated carbon) can be used in filtration and is effective in improving the taste, odour and colour of the water. This system uses a bed of activated carbon to remove contaminants and impurities.
Activated carbon works via a process called adsorption, whereby pollutant molecules in the fluid to be treated are trapped inside the pore structure of the carbon substrate. Each particle, or granule, of carbon provides a large surface area, or pore structure, allowing contaminants the maximum possible exposure to the active sites within the filter media. One gram of activated carbon has a surface area in excess of 3,000 m2 (32,000 sq ft).
Activated charcoal is charcoal that has been treated with oxygen to open up millions of tiny pores between the carbon atoms.
Adsorption is important here. When a material adsorbs something, it attaches to it by chemical attraction. The huge surface area of activated charcoal gives it countless bonding sites. When certain chemicals pass next to the carbon surface, they attach to the surface and are trapped.
Activated charcoal is good at trapping other carbon-based impurities (“organic” chemicals), as well as things like chlorine. Many other chemicals are not attracted to carbon at all (sodium, nitrates, etc.) so they pass right through. This means that an activated charcoal filter will remove certain impurities while ignoring others. It also means that, once all of the bonding sites are filled, an activated charcoal filter stops working.
Charcoal filter should be replaced regularly, because bacteria can breed in it.
Fine Sand Filtration (slow)
 In slow sand filtration the water passes slowly downwards, through a thick bed of fine sand. For the filter to perform well there should be no sudden changes in the flow rate and the water should not be very turbid (cloudy with suspended solids), or the filter will quickly become blocked. Good slow sand filters can produce good quality drinking-water.
In slow sand filtration the water passes slowly downwards, through a thick bed of fine sand. For the filter to perform well there should be no sudden changes in the flow rate and the water should not be very turbid (cloudy with suspended solids), or the filter will quickly become blocked. Good slow sand filters can produce good quality drinking-water.
Biological layer (Biosand)
There are a number of processes which improve the water quality as it passes through the filter, but pathogens are mainly removed in the very top layer of the filter bed where a biological film (called the ‘schmutzdecke’) builds up. In a welldesigned and well-operated filter this film strains out bacteria. Deeper in the sand bed, bacteria that pass through the schmutzdecke are killed by other micro-organisms, or they become attached to particles of sand until they die.
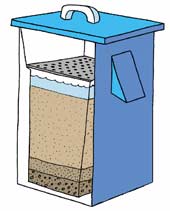
The water level can be maintained at 5-6 centimeters above the sand layer by setting the height of the outlet pipe. This shallow water layer allows the bioactive layer to grow on top of the sand, which helps reduce disease-causing organisms. A plate with holes in it is placed on the top of the sand to prevent disruption of the bioactive layer when water is added to the system.
The schmutzdecke takes time to become effective, so water needs to flow though a new filter for at least a week before the filter will work efficiently. The raw water should contain a fair amount of oxygen (Aeration) to promote the useful biological activity both in the schmutzdecke and further down into the filter bed.
After a period of use, the material filtered out of the water blocks the surface of the sand and reduces the flow rate to an unacceptable level. When this happens the filter is drained to expose the sand, and the top 15 to 20mm of the bed is carefully removed. When the filter is restarted, it takes a few days before the schmutzdecke builds up again to provide good quality water so, during this period, the water should not be used for potable purposes.
When successive cleaning operations cause the depth of sand to reach the minimum acceptable value (conventionally about 65cm thick), additional clean sand needs to be added to the bed.
Ceramic Pot Filtration
 The ceramic filter is a micro-porous ‘flower pot shaped’ device (various types of clay, carved porous stone, diatomaceous earth,…) that holds a limited amount of water. Ceramic filters have traditionally been used for water treatment throughout the world. Currently, the most widely distributed ceramic filter is the Potters for Peace (PFP) filter, which is shaped like a flowerpot and impregnated with colloidal silver.
The ceramic filter is a micro-porous ‘flower pot shaped’ device (various types of clay, carved porous stone, diatomaceous earth,…) that holds a limited amount of water. Ceramic filters have traditionally been used for water treatment throughout the world. Currently, the most widely distributed ceramic filter is the Potters for Peace (PFP) filter, which is shaped like a flowerpot and impregnated with colloidal silver.
Laboratory testing has shown that although the majority of the bacteria are removed mechanically through the filter’s small (0.6 – 3.0 microns) pores, coating with colloidal silver is necessary to inactivate 100% of the bacteria. The filter removes 99.99% of protozoa by mechanical processes; however, the effectiveness of the filter in inactivating or removing viruses is yet to be proved.
Their effectiveness depends on the size of the pores in the clay or other material. Good quality filters have micron or submicron pore sizes. Pathogens and suspended material are trapped on the ceramic material as water is poured through the filter. If properly constructed and operated, a ceramic filter can be very effective in producing good quality water.
The impurities are deposited on the surface of the filter, so need to be regularly scrubbed off to maintain a good flow rate. The filter should be cleaned when the flow-rate is reduced or stops, or when any plastic part becomes visibly dirty.
With a low cost worldwide and health impacts similar to other treatment options, the cost effectiveness of ceramic filters compares well with other Household Water Treatment Systems.
Chlorine Disinfection
 Chlorine is widely used across the world as a disinfectant as it is inexpensive, widely accessible while being easy to control and monitor. It is highly effective at eradicating waterborne diseases and killing pathogens. Therefore, this powerful disinfectant is popularly used in municipal water treatment plants to eliminate bacteria and other potential harmful microorganisms.
Chlorine is widely used across the world as a disinfectant as it is inexpensive, widely accessible while being easy to control and monitor. It is highly effective at eradicating waterborne diseases and killing pathogens. Therefore, this powerful disinfectant is popularly used in municipal water treatment plants to eliminate bacteria and other potential harmful microorganisms.
Disinfecting water with chlorine will kill any bacteria and viruses (although it does not deactivate some parasites like giardia, cryptosporidium and worm eggs). Chemical disinfection using chlorine has the benefits of being relatively quick, simple, and cheap and allows a residual amount of chlorine to remain in the water to provide some protection against subsequent contamination.
Chlorine is also an oxidizer, so it helps to remove iron, hydrogen sulfide, and other minerals. However, despite its effectiveness and its ability to disinfect drinking water, chlorination is a problem on its own and has several negative health effects.
There are several different sources of chlorine for home use including liquids (such as bleach), powders (such as bleaching powder) and purpose-made tablets. Iodine, another chemical disinfectant, is also used sometimes. The strength of the chlorine compounds will vary with time; air-tightness, low temperature and absence of light are particularly important during storage.
Chlorine compounds usually destroy pathogens after 30 minutes of contact time, and free residual chlorine (0.2-0.5 mg per litre of treated water) provide ongoing disinfection. Several chlorine compounds, such as sodium hypochlorite and calcium hypochlorite, can be used domestically, but the active chlorine concentrations of such sources can be different and this should be taken into account when calculating the amount of chlorine to add to the water. The amount of chlorine that will be needed to kill the pathogens will be affected by the quality of the untreated water and by the strength of the chlorine compound used. If the water is excessively turbid, it should be filtered or allowed to settle before chlorinating it.
To Disinfect water using household bleach, if you can’t boil water. Only use regular, unscented chlorine bleach products that are suitable for disinfection and sanitization as indicated on the label. The label may say that the active ingredient contains 6 or 8.25% of sodium hypochlorite. Do not use scented, color safe, or bleaches with added chemicals.
− If water is cloudy, let it settle and filter it through a clean cloth, paper towel, or coffee filter.
− Locate a clean dropper from your medicine cabinet or emergency supply kit.
− Locate a fresh liquid chlorine bleach that is stored at room temperatures for less than one year.
− Follow the product’s label indication to decide how many drops of bleach you should add to the water. Commonly 8 drops of 6 % sodium hypoclhorite bleach or 6 drops of 8.25% bleach are used for every gallon of water.
− Stir and let stand for 30 minutes for the chemicals to have sufficient contact time with the pathogens. The water should have a slight chlorine odor. If it doesn’t, repeat the dosage and let stand for another 15 minutes before use.
− If the chlorine taste is too strong, use the Aeration technique, pour the water from one clean container to another and let it stand for about an hour (preferably in the sun) before use. UV rays from the sun will rapidly break down chlorine molecules. Another option good at removing chlorine is to use a carbon filter.
Solar Disinfection (SODIS)
 SODIS is a really simple and low-cost technology that uses a combination of solar UV radiation and increased temperatures to destroy bacteria, viruses, and protozoa (including cryptosporidium and giardia) present in water. Its efficiency depends on the water temperature reached during solar exposure.
SODIS is a really simple and low-cost technology that uses a combination of solar UV radiation and increased temperatures to destroy bacteria, viruses, and protozoa (including cryptosporidium and giardia) present in water. Its efficiency depends on the water temperature reached during solar exposure.
The SODIS system uses transparent bottles which are halfblackened to collect and radiate heat, with the clear side of the bottle facing the sun. For greater effectiveness place the bottle (black side down) on a corrugated-iron roof. The water can also be held in a plastic bag.
Waterborne microbes are thus inactivated through the combined germicidal effects of both UV radiation and heating of the sunlight. In this type of bottle, water can be heated to temperatures of 50-55°C and even higher (60°C) when exposed to sunlight for several hours. Microbes differ in sensitivity to inactivation by heat and by UV radiation. A complementary effect exists thus between the two ways of action. Direct radiation of sunlight works synergistically with solar heating of the water to disinfect the water.
Thermal inactivation is found to be important only at water temperatures above 45-50°C, at which point strong synergy between optical and thermal inactivation processes is observed. Therefore, achieving sufficiently high temperatures (50°C and higher for several hours) is an important factor for microbial inactivation by solar disinfection systems.
In tropical regions, a safe exposure period is about five hours, centred around midday.
Vigorously shaking bottles which are filled to ¾ of their capacity to increase the oxygen content of the water (Aeration) before exposing it to sunlight considerably improves the effectiveness of solar disinfection (Reed 1997). Further sporadic shaking during exposure is also beneficial.
Users of SODIS fill plastic soda bottles with low-turbidity water, shake them to oxygenate the water, and place the bottles on a roof or rack for six hours (if sunny) or two days (if cloudy). SODIS has been proven to inactivate bacteria and viruses; the protozoa cryptosporidium and giardia are also sensitive to solar irradiation.
If when one’s hand is placed behind a full bottle laying horizontally and the fingers are still visible, then the turbidity requirement is satisfied and water can be adequately treated. Pretreatment to reduce turbidity is needed if fingers cannot be seen.
Clean the bottles with soap and a bottle brush if available if you observe the formation of algae on the inner side of the bottle. Replace bottles when heavily scratched, opaque, or leaking.
Choosing the right container for SODIS
There are a few simple criteria that must be applied in selecting the appropriate type of containers to be used for the proper disinfection of contaminated drinking water by sunlight. The general rule that needs to be followed is to use containers that would permit the penetration of those sun rays that would effectively destroy microorganisms. Therefore, the transparency and colour of the materials from which the containers are made constitute two important characteristics.
Colourless plastic or glass containers are the best choice whenever available. This is because they transmit light in the nearultraviolet region (A), which is the most lethal region as it accounts for about 70% of the bacterial destruction potential, as well as in the visible range of the spectrum. Violet and blue tinted containers come next in the order of priority. Since the lethal action continues to decrease thereafter in the descending order of green > yellow > orange > red, containers with these colours should be avoided. Very light green containers may be used provided the period of exposure to sunlight is somewhat extended.
Stated differently, containers made of plastic or glass with green, yellow, orange, or red colours obstruct the transmission of the most lethal rays of sunlight, unlike those that are colourless or blue. Therefore, preference should be given to containers that are either colourless or blue.
The wall thickness of the containers is another factor that needs to be considered. The thicker the wall of a container the less the transmission of the effective UV rays of sunlight. This would in turn somewhat retard the disinfection process, thus requiring a longer solar exposure period.
Boiling
 Boiling is a very simple method of water disinfection sufficient to kill pathogenic bacteria, viruses and protozoa (WHO, 2015). Heating water to a high temperature, 100°C, kills most (many are killed at 70°C) of the pathogenic organisms, particularly viruses and bacteria causing waterborne diseases.
Boiling is a very simple method of water disinfection sufficient to kill pathogenic bacteria, viruses and protozoa (WHO, 2015). Heating water to a high temperature, 100°C, kills most (many are killed at 70°C) of the pathogenic organisms, particularly viruses and bacteria causing waterborne diseases.
Water should be brought to a ‘rolling boil‘ for 1 minute. The recommended boiling time is one minute at sea level, adding one minute for every additional 1000 meters (5,000 feet) in altitude.
The main disadvantages of boiling water are that it uses up fuel and it is time consuming. It also affects the taste of the water, although increasing the air content (Aeration) by vigorously stirring the water, or shaking it in a bottle, after cooling, will improve the taste.
Effective in destroying all classes of waterborne pathogens (viruses, bacteria, bacterial spores, fungi, protozoan, helminth ova), if correctly applied in terms of temperature and time.
• Can be effectively applied to all waters, including those with high turbidity or dissolved constituents.
• Rolling boil = indication easily identified (when temperature sensors are not available) of the efficiency of the treatment.
− If water is cloudy, let it settle and filter it through a clean cloth, paper towel, or coffee filter.
− Bring water to a rolling boil for at least one minute. At altitudes above (1,000 meters), boil water for three minutes.
− Let water cool naturally and store it in clean containers with covers.
− To improve the flat taste of boiled water, add one pinch of salt to each quart or liter of water, or pour the water from one clean container to another several times (Aeration technique).
Solar Water Distillation
 Solar Stills operate on the same principles that produce rainfall. It consists of a food grade black silicone container where impure water collects at shallow depth. The container is covered with a sloping glass cover, all sealed tightly to prevent the entrance of fresh air into the unit. This container can be supported by a suitable insulating wooden frame.
Solar Stills operate on the same principles that produce rainfall. It consists of a food grade black silicone container where impure water collects at shallow depth. The container is covered with a sloping glass cover, all sealed tightly to prevent the entrance of fresh air into the unit. This container can be supported by a suitable insulating wooden frame.
The impure water absorbs solar radiation and becomes heated. Increasing water temperature stimulates water molecules to evaporate. When the vapor comes into contact with the cool inner surface of the transparent glass cover, condensation occurs. This condensate water slips down, accumulates in a trough along the lower side of the glass cover and moves through a tube out of the enclosure, where pure desalinated water is collected.
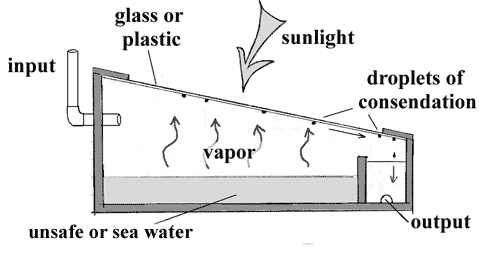
Water Distillation produces water without chemical salts and minerals. Distillation is also effective in removing other chemicals like fluoride, arsenic and iron. The water produced is relatively tasteless unless a little salt is added.
Various methods can be used to distill water at household level, for example to treat sea water.
In an emergency situation, furniture drawers can be used for the container structure, which will be lined with the black plastic of large waste bags. Paperboard from used boxes can be attached to both sides to complete the structure, replacing the glass with a food grade transparent kitchen roll typically found in our kitchen.




